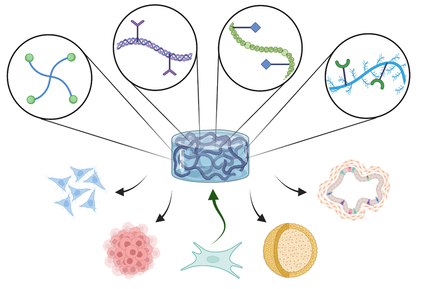
Biomimetic & dynamic hydrogels
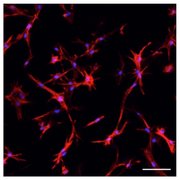
Hydrogels with spatial-temporally tunable properties are increasingly used in tissue engineering and regenerative medicine applications. Utilizing a combination of functionalized synthetic/natural macromers, we aim to demonstrate how synthetic and biomimetic hydrogels can be engineered with highly defined and tunable physicochemical properties to direct cell fate processes in vitro. Photochemistry and biochemical reactions are used together to prepare cell-laden gels with biomimetic, responsive, and viscoelastic properties, as well as gels with different architectures, including cell surface coating, multi-layer bulk hydrogels, and microgels.
Artificial stem cell niche
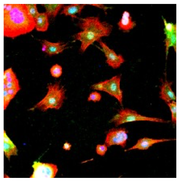
Human induced pluripotent stem cell (hiPSC) technology has the potential to provide unlimited cells for basic sciences and translational medicine applications, including treatments of type 1 diabetes. However, current strategies for in vitro differentiation of hiPSCs into insulin-secreting cells have primarily focused on adding soluble factors to cells cultured on two-dimensional (2D) substrates or using three-dimensional (3D) cell-laden matrices with static or undefined properties. In this project, we are developing accessible synthetic and biochemical strategies to create developmentally-inspired hydrogels. These gels will possess unprecedented tunability in matrix biophysical and biochemical properties for enhancing pancreatic differentiation of hiPSC.
Cancer bioengineering
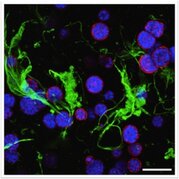
Cancer progression is guided by complex cell-matrix and cell-cell interactions. We are particularly interested in developing technology to study pancreatic cancer as many anti-pancreatic cancer therapeutics have been identified and tested, but very few have progressed to clinical success. Studying the complex cellular and molecular event in stromal tissues (i.e., desmoplasia) requires a well-defined and tunable 3D cell-laden matrix. The objective of this project is to use adaptable matrices with spatial-temporally tunable properties for elucidating the molecular mechanisms governing pancreatic cancer cell progression and for identifying novel molecular targets against this deadly disease. We are also developing convenient hypoxia-inducible hydrogels for recreating the hypoxic microenvironment of a tumor niche. Additional efforts are devoted to synthesizing injectable nanoparticle-loaded matrices for delivering multiple anti-tumor or other therapeutic agents.
Pancreatic & liver tissue engineering
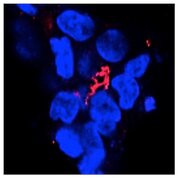
In the area of pancreatic tissue engineering, we are developing biomimetic conformal coatings and injectable matrices to eliminate immuno-suppressive regimens in pancreatic islet transplantation. This new technique will improve the design of hydrogel conformal coating while allowing facile conjugation of a myriad of bioactive motifs to improve the survival and function of transplanted islets for effective treatment of type 1 diabetes. In the area of liver tissue engineering, we are using the various biomimetic hydrogel formulations available in our lab to differentiate hepatic progenitor cells into functional hepatocytes for the purpose of mechanistic understanding of hepatocyte proliferation and function.
Hydrogels with spatial-temporally tunable properties are increasingly used in tissue engineering and regenerative medicine applications. Utilizing a combination of functionalized synthetic/natural macromers, we aim to demonstrate how synthetic and biomimetic hydrogels can be engineered with highly defined and tunable physicochemical properties to direct cell fate processes in vitro. Photochemistry and biochemical reactions are used together to prepare cell-laden gels with biomimetic, responsive, and viscoelastic properties, as well as gels with different architectures, including cell surface coating, multi-layer bulk hydrogels, and microgels.
Artificial stem cell niche
Human induced pluripotent stem cell (hiPSC) technology has the potential to provide unlimited cells for basic sciences and translational medicine applications, including treatments of type 1 diabetes. However, current strategies for in vitro differentiation of hiPSCs into insulin-secreting cells have primarily focused on adding soluble factors to cells cultured on two-dimensional (2D) substrates or using three-dimensional (3D) cell-laden matrices with static or undefined properties. In this project, we are developing accessible synthetic and biochemical strategies to create developmentally-inspired hydrogels. These gels will possess unprecedented tunability in matrix biophysical and biochemical properties for enhancing pancreatic differentiation of hiPSC.
Cancer bioengineering
Cancer progression is guided by complex cell-matrix and cell-cell interactions. We are particularly interested in developing technology to study pancreatic cancer as many anti-pancreatic cancer therapeutics have been identified and tested, but very few have progressed to clinical success. Studying the complex cellular and molecular event in stromal tissues (i.e., desmoplasia) requires a well-defined and tunable 3D cell-laden matrix. The objective of this project is to use adaptable matrices with spatial-temporally tunable properties for elucidating the molecular mechanisms governing pancreatic cancer cell progression and for identifying novel molecular targets against this deadly disease. We are also developing convenient hypoxia-inducible hydrogels for recreating the hypoxic microenvironment of a tumor niche. Additional efforts are devoted to synthesizing injectable nanoparticle-loaded matrices for delivering multiple anti-tumor or other therapeutic agents.
Pancreatic & liver tissue engineering
In the area of pancreatic tissue engineering, we are developing biomimetic conformal coatings and injectable matrices to eliminate immuno-suppressive regimens in pancreatic islet transplantation. This new technique will improve the design of hydrogel conformal coating while allowing facile conjugation of a myriad of bioactive motifs to improve the survival and function of transplanted islets for effective treatment of type 1 diabetes. In the area of liver tissue engineering, we are using the various biomimetic hydrogel formulations available in our lab to differentiate hepatic progenitor cells into functional hepatocytes for the purpose of mechanistic understanding of hepatocyte proliferation and function.
External funding agencies